Graphite bipolar plate
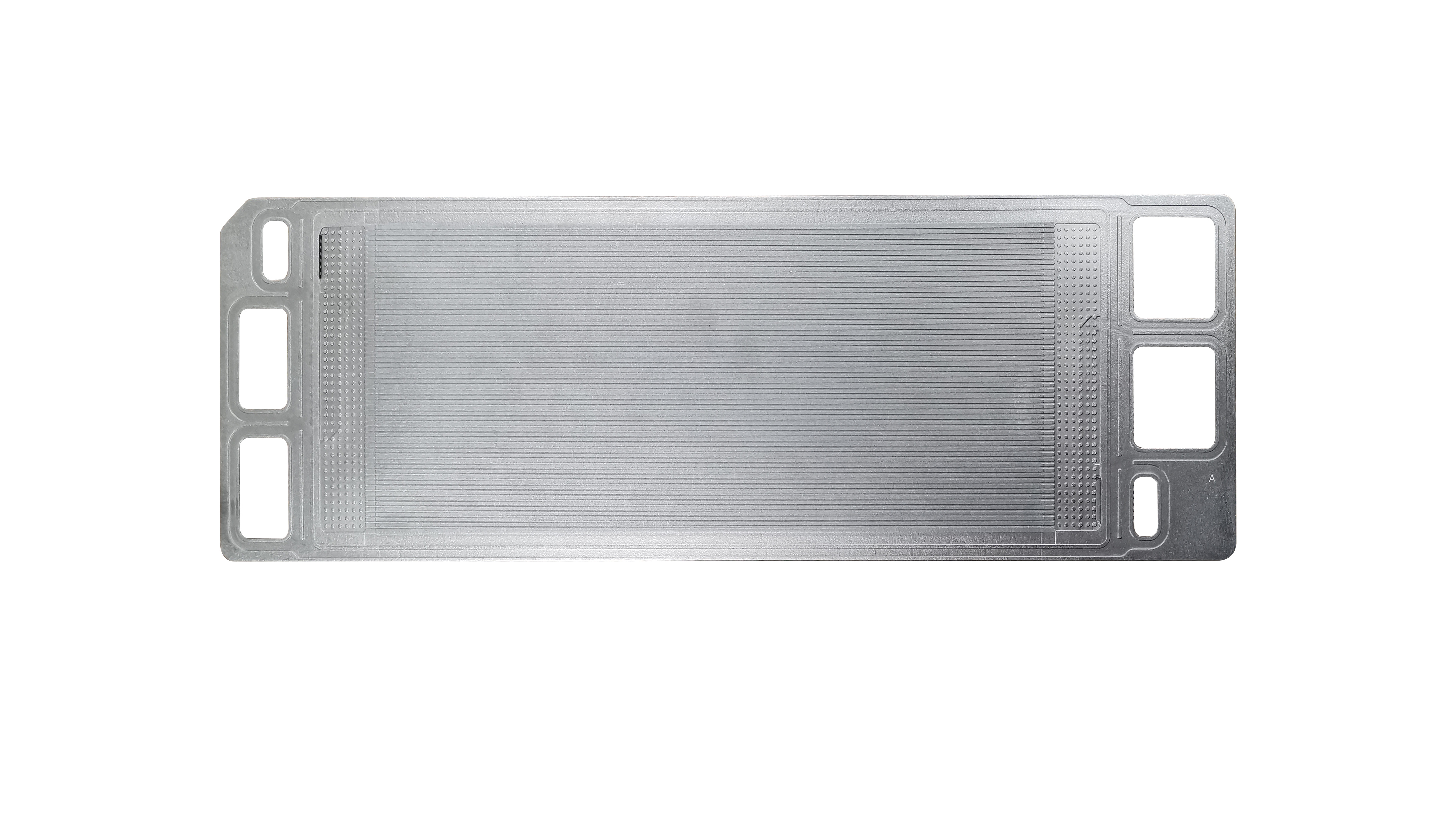

Graphite bipolar plates play a crucial role in vanadium redox flow batteries. They are responsible for collecting and conducting current during the battery's charge and discharge processes, providing effective mechanical support for the membrane and electrodes, connecting multiple single cells, and preventing the mixing of positive and negative electrolyte and direct contact between the electrodes. Due to their good conductivity, low density, and excellent corrosion resistance, graphite bipolar plates are widely used in vanadium redox flow batteries.
Product advantages
- Good electrical conductivity: Graphite materials have excellent electrical conductivity, which allows graphite bipolar plates to effectively collect and conduct current.
- Excellent corrosion resistance: Graphite bipolar plates exhibit good corrosion resistance in the acidic environment of all-vanadium flow batteries, which is crucial for the long-term stable operation of the battery. The corrosion resistance of graphite bipolar plates is superior to that of metal bipolar plates, which are prone to corrosion in acidic environments, leading to performance degradation.
- Lower density: Compared to metal materials, graphite has a lower density, which helps reduce the overall weight of the battery and improve its energy density.
- Cost-effectiveness: Graphite materials are relatively low-cost compared to metal materials, making graphite bipolar plates more cost-effective for large-scale production and application.
- Environmental adaptability: Graphite bipolar plates can maintain stability within the operating temperature range of all-vanadium flow batteries, adapting to different environmental conditions.
- Plasticity: Graphite bipolar plates can be manufactured into the desired shapes and sizes through various processing methods to meet different battery design requirements.

For more information, please continue to visit the subsidiary website



Address: No. 66, Zhengjia Industrial Park, Liaocheng City, Shandong Province

Phone: +86-0635-8683098

Email: salesplan@gebchina.cn
Copyright © Shandong Golden Empire Precision Machinery Technology Co., Ltd
Statement
Privacy Policy
Terms of Use
Cookie Policy